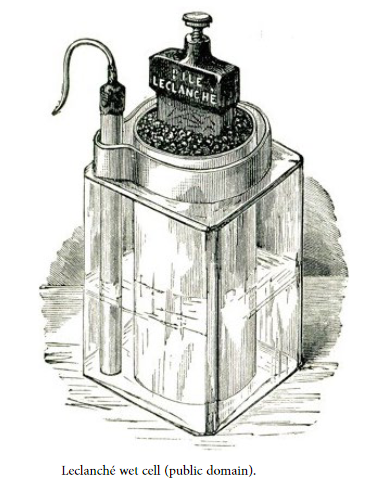
A dry cell is a type of electrochemical cell that was developed in the 19th century. A dry cell uses a chemical reaction between the electrodes and the electrolyte. These cells consist of low-moisture electrolytes in a paste form (providing just enough moisture to allow the current to flow) combining carbon and zinc ions; dry cells are also known as Leclanché cells.
While the Zamboni pile, introduced in 1812, is considered the first example of a dry cell, due to its capacity limitations it never gained traction. Introduced in France in 1866, the Leclanché cell (or zinc-carbon cell) is the precursor of our modern alkaline batteries. The first significant dry cell however was introduced by the German Carl Gassner in 1886, who used zinc as the container to house the cell’s other components while also using the sealed zinc container as the anode.
Dry cells are typically used in portable electronic devices and smaller home appliances – the most common dry cell battery is the AA battery that almost everyone has used at some point in their lives. They’re also used in most cars today, as they’re considered more environmentally friendly compared to their wet cell equivalents since there’s no risk of acid fumes or liquids (acids) coming out from them. Tesla’s car batteries, for example, use 4680 dry cell batteries bundled together.
Dry cell batteries are the most commonly used batteries today and, as mentioned above, they greatly vary in size. They are also lighter than the wet cell batteries, easy to bundle together to produce more electricity, and easier to “maintain”, since there’s no need to constantly check the electrolyte level in them. These details are what made the dry cell battery so successful when it emerged; unlike the cumbersome cells with liquid electrolytes, it allowed easy transportation, storage, and universal deployment in any orientation (quite an achievement at the time). Put differently, the dry cell battery is one of the defining inventions of the 20th century.
An interesting fact that not many people know is that the AA batteries are by far the most common type of alkaline household batteries. Another interesting one is how old AA batteries are; they were introduced in 1907, 7 years before the WW1, and only standardised 40 years later, in 1947, after WW2.
Similar to alkaline cell batteries, a dry cell battery does not deliver a high voltage. The maximum level for a dry cell battery is 1.5 volts.
Primary and secondary dry cell batteries
Dry cell batteries can be classified as either primary or secondary. Primary batteries are single-use (non-rechargeable), where the electrochemical reactions consume all the reagents, whilst secondary ones can be recharged via battery rechargers that are able to regenerate the required chemical reactions.
Primary (single-use) batteries
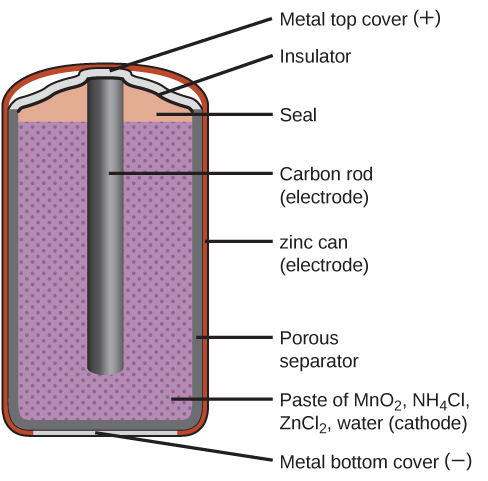
The majority of alkaline batteries on the market today are primary dry cell batteries; two of their main advantages is that they’re inexpensive and easy to use. The alkaline batteries were developed in the 1950s as replacements for the zinc-carbon dry cells, which are still used today. However, not all single-use batteries are alkaline or zinc-carbon, some are mercury-based, silver-oxide, zinc air, or lithium-based batteries. In many cases these need to be handled differently than the alkaline batteries and have specialised shapes for specific equipment, which should make it a bit easier for the end consumer to distinguish them from the alkaline or zinc-carbon batteries. Think of the button-cell (coin-shaped) batteries, for example.
Single-use batteries (also called household batteries) are the most common consumer-grade batteries and include these models: AA, AAA, D-Cell, C-cell, and 9 volts. They’re used for a wide variety of products, such as torches, bike lights, portable AM/FM radios, remote controls, children toys, medical devices, cameras, alarm clocks, wristwatches, calculators, and many more.
Advantages and disadvantages of the dry cell batteries
Advantages:
- Wide availability: availability in most places and can be purchased easily.
- Portability: they are small and light, perfect for portable electronic devices.
- Low maintenance: do not require regular refilling with electrolytes such as with other types of batteries.
- Long shelf life: they can be stored for long periods of time without losing charge.
- Safe: less likely to leak, making them much safer than other batteries.
Disadvantages:
- Limited capacity: shorter lifespan (not shelf life) so need to be replaced more frequently.
- Low energy density: may not last as long or provide as much power as other batteries of the same size.
- Disposal: contain toxic chemicals that can harm the environment if they are not disposed of properly. In fact, every year more than 1.9 million tons of discarded batteries are produced and only 48% is reprocessed.
- Temperature sensitivity: can lose their charge or even leak if exposed to high temperatures or freezing for extended periods of time.
- Cost: relatively expensive compared to other types of batteries, especially if you need to replace frequently.

One Reply to “Dry cell batteries advantages & disadvantages”
Thank you for this help so much it really helps