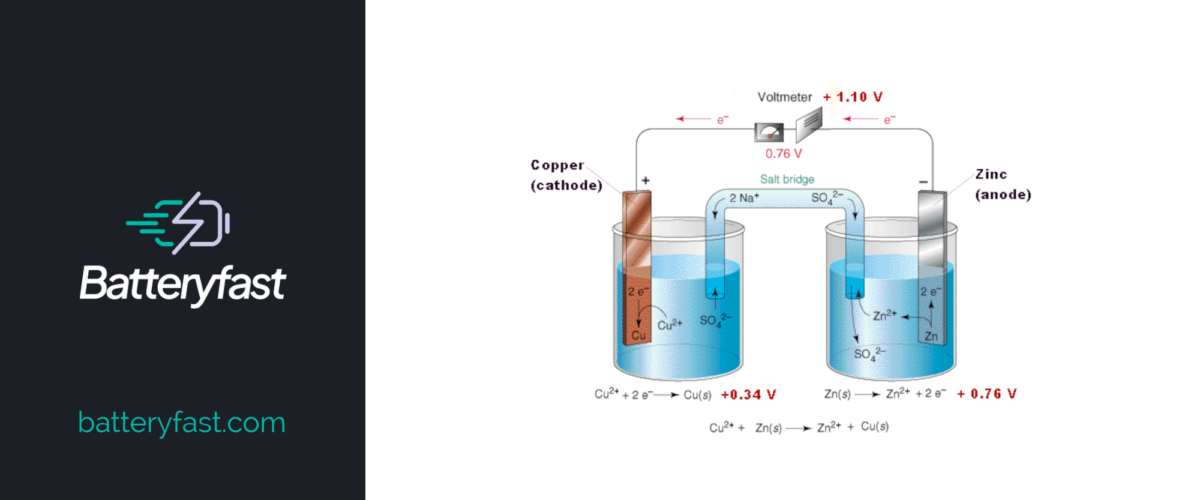
A galvanic cell is a specific type of electrochemical cell that is commonly used to supply electric current. Named after the renowned scientists Luigi Galvani and Alessandro Voltais, it does this by transferring electrons via a redox reaction. A redox reaction is a type of chemical reaction that involves the transferring of electrons between two species. The word ‘redox’ is actually the abbreviation of reduction-oxidation. These reactions occur simultaneously.
This is an excellent example of the way in which energy can be harnessed courtesy of reactions between just a few specific elements.
Galvanic cells can be made out of any two metals that form the anode and the cathode. In the galvanic cell, the anode is where the oxidation happens, meaning it is the negative electrode. The cathode is, therefore, positive.
The main differences between galvanic cells and electrolytic cells are that the galvanic cells are able to produce electric current using chemical reactions that spontaneously occur, whilst electrolytic cells do the opposite i.e., use electric current from an external source to create chemical reactions.
The main application of galvanic cells is in batteries (notably 1.5V alkaline batteries), but they are also found in rechargeable devices and nowadays in electric cars.