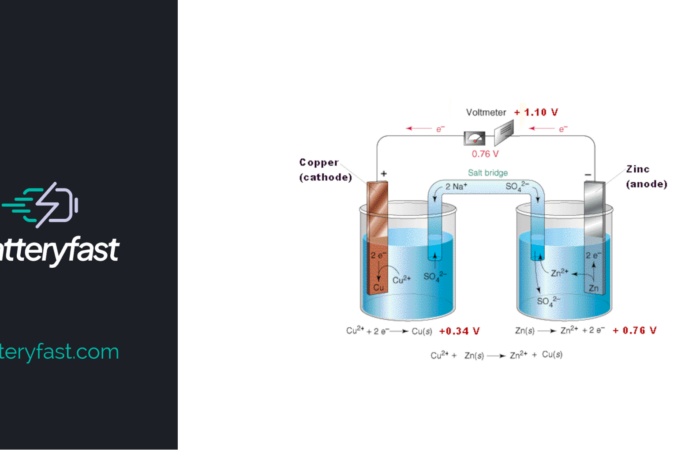
Galvanic Cell
A galvanic cell is a specific type of electrochemical cell that is commonly used to supply electric current. Named after the renowned scientists Luigi Galvani and Alessandro Voltais, it does this by transferring electrons via a redox reaction. A redox reaction is a type of chemical reaction that involves the transferring of electrons between two […]